Sulfonic Acid: Uses, Structure & Synthesis
High-Purity Sulfonic Acid for Industrial and Specialty Applications
Sulfonic Acid: Uses, Structure & Synthesis
Sulfonic acid is one of the most important classes of organic acids in industrial and applied chemistry. From detergents and dyes to pharmaceuticals and catalysts, sulfonic acid derivatives play a critical role in modern chemical manufacturing. This article explains what sulfonic acid is, its chemical structure, methods of synthesis, and its real-world applications, using only validated and authoritative sources.
What Is Sulfonic Acid
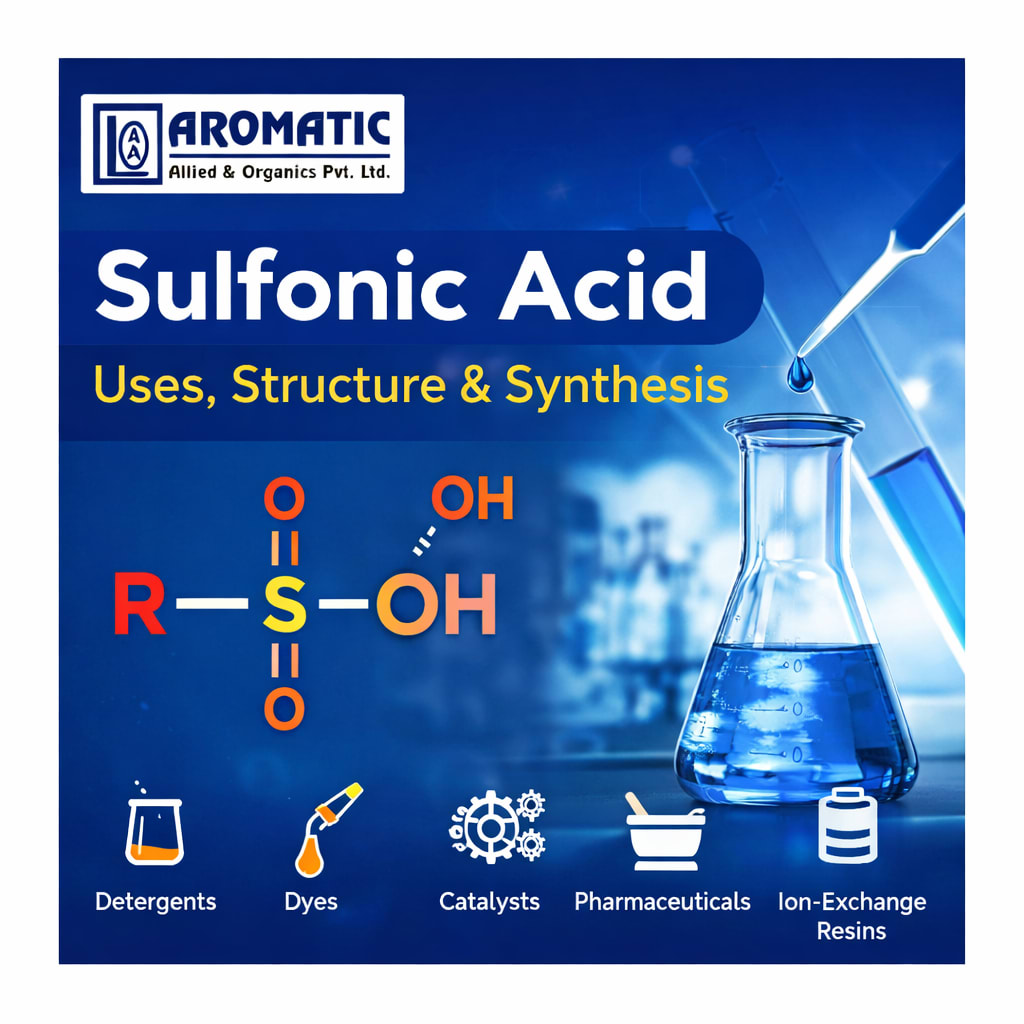
Sulfonic acids are a group of organosulfur compounds characterized by the functional group –SO₃H, where the sulfonyl group is directly bonded to a carbon atom of an organic moiety (R–SO₃H).
According to Encyclopaedia Britannica, sulfonic acids are among the strongest organic acids, with acid strength comparable to mineral acids such as hydrochloric acid. Their strength arises from the high stability of the sulfonate anion formed after proton dissociation.
Unlike carboxylic acids, sulfonic acids:
- Are almost completely ionized in water
- Exhibit high thermal and chemical stability
- Form highly soluble salts known as sulfonates
Chemical Structure of Sulfonic Acid
- The sulfonic acid functional group consists of:
- One sulfur atom at the center
- Three oxygen atoms bonded to sulfur
- One hydroxyl group (–OH)
- One carbon–sulfur bond connecting the group to an organic structure
The sulfur atom adopts a tetrahedral geometry, and the conjugate base (sulfonate ion, RSO₃⁻) is stabilized by resonance across the three oxygen atoms. This extensive delocalization of negative charge explains the strong acidity of sulfonic acids.
Because of this structure:
- Sulfonic acids are stronger than carboxylic acids
- Their salts remain stable across wide pH ranges
- They remain highly soluble in aqueous systems
Synthesis of Sulfonic Acid
1. Sulfonation Reaction (Primary Industrial Method)
The most common method for synthesizing sulfonic acids is sulfonation, where an organic compound reacts with sulfur trioxide (SO₃), concentrated sulfuric acid, or oleum.
In aromatic systems such as benzene:
- Sulfur trioxide acts as an electrophile
- The reaction introduces the –SO₃H group directly onto the aromatic ring
- The process is reversible under certain conditions
This method is widely used to produce:
- Benzene sulfonic acid
- Alkylbenzene sulfonic acids used in detergents
2. Alternative Synthetic Routes
Other validated methods include:
- Oxidation of thiols or sulfides to sulfonic acids
- Reaction of alkyl halides with sulfite salts followed by oxidation
- Use of chlorosulfonic acid for controlled sulfonyl group introduction
These methods are typically applied in laboratory or specialty chemical synthesis rather than bulk production.
Uses of Sulfonic Acid
1. Detergents and Surfactants
Sulfonic acids form the backbone of the modern detergent industry. Linear alkylbenzene sulfonic acid (LABSA) is one of the most widely used surfactant intermediates globally.
Key properties that make sulfonic acids ideal for detergents:
- Strong hydrophilic behavior
- Excellent emulsification capacity
- High stability in hard water
2. Dyes and Pigments
Sulfonic acid groups increase the water solubility of dyes and improve their binding affinity to fibers. They are essential in:
- Azo dyes
- Anthraquinone dyes
- Textile and printing inks
3. Catalysts in Organic Synthesis
Strong sulfonic acids such as:
- p-Toluenesulfonic acid (PTSA)
- Methanesulfonic acid
Are widely used as non-oxidizing acid catalysts in esterification, alkylation, and polymerization reactions.
Their advantage lies in high acidity combined with lower corrosion compared to mineral acids.
4. Pharmaceuticals and Fine Chemicals
Sulfonic acid chemistry underpins the synthesis of:
- Sulfonamide drugs
- Active pharmaceutical intermediates
- Ionic drug salts for enhanced bioavailability
5. Ion-Exchange Resins and Polymers
Sulfonic acid groups are chemically bound to polymer backbones to create cation-exchange resins, widely used in:
- Water softening
- Fuel cells
- Chemical separation technologies
Key Properties of Sulfonic Acids
- Very strong organic acids
- High water solubility
- Excellent thermal and chemical stability
- Form stable sulfonate salts
- Non-oxidizing behavior in many derivatives
These properties explain their dominance in industrial chemistry.
Why Sulfonic Acid Matters in Modern Industry
Sulfonic acid is not a niche chemical class. It is foundational. Without sulfonic acids, modern detergents, textile dyes, catalytic processes, and ion-exchange systems would not function at current efficiency levels. Their unique combination of acid strength, stability, and versatility makes them indispensable across multiple sectors.
About the Creator
Shraddha Mahajan
I am a content marketing virtuoso, writing for the home decor niche, who travels the world, delivering global touches of elegance and inventiveness in every tale enriched by this vivid mosaic of my international life.






Comments
There are no comments for this story
Be the first to respond and start the conversation.