Formulation Development Outsourcing Market Size and Forecast 2025–2033
Why pharmaceutical companies are increasingly turning to CDMOs to accelerate innovation, control costs, and navigate regulatory complexity
Introduction: A Market Built on Speed, Science, and Specialization
The global pharmaceutical industry is in the middle of a profound transformation. Drug pipelines are becoming more complex, regulatory expectations more demanding, and development timelines more compressed. In this environment, the Formulation Development Outsourcing Market is emerging as a critical enabler of innovation and efficiency.
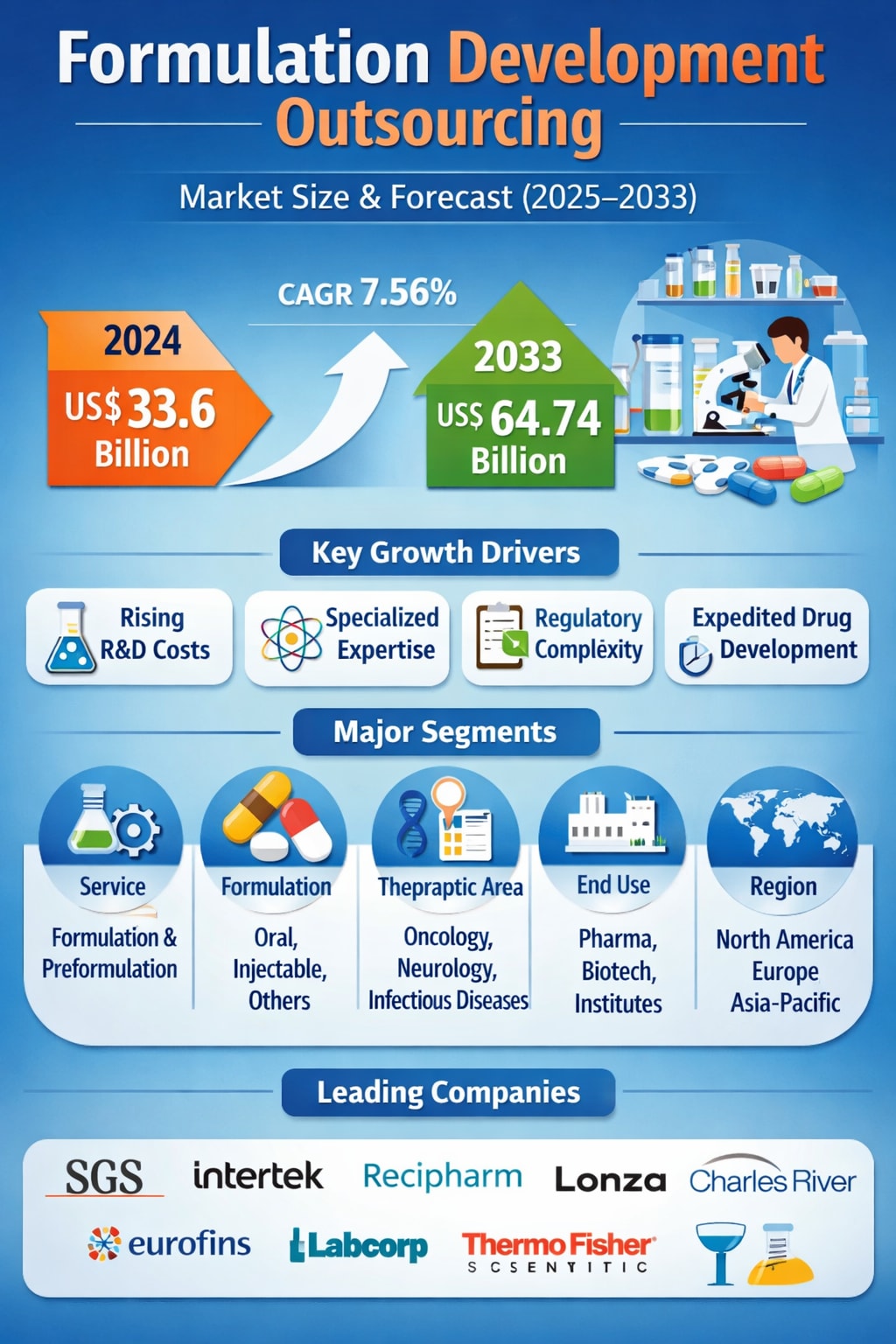
According to Renub Research, the Formulation Development Outsourcing Market is expected to reach US$ 64.74 billion by 2033, up from US$ 33.6 billion in 2024, growing at a compound annual growth rate (CAGR) of 7.56% from 2025 to 2033. This strong growth reflects a broader industry shift: pharmaceutical and biopharmaceutical companies are increasingly relying on specialized external partners to manage the scientific, technical, and regulatory complexities of modern drug formulation.
Formulation development is no longer just about turning an active ingredient into a pill or injection. Today, it involves advanced work on bioavailability, stability, controlled release, patient compliance, and manufacturability. As therapies become more sophisticated—particularly in oncology, neurology, and biologics—the need for deep, niche expertise has made outsourcing not just attractive, but often essential.
Global Formulation Development Outsourcing Market Overview
The global formulation development outsourcing market is expanding rapidly due to the growing complexity of pharmaceutical products and the rising need for cost-effective drug development models. Pharmaceutical and biotechnology companies are under pressure to reduce time-to-market, control R&D spending, and meet strict regulatory standards—all while maintaining high quality and safety benchmarks.
To meet these demands, many companies are turning to Contract Development and Manufacturing Organizations (CDMOs) and specialized service providers. These partners offer a wide range of services, from preformulation studies and stability testing to full-scale formulation development and regulatory support. This approach is especially valuable for small and mid-sized companies that lack extensive in-house R&D infrastructure.
Another key growth driver is the rising number of complex, biologic, and generic drugs entering development pipelines. These products often require customized formulation strategies, advanced analytical capabilities, and specialized manufacturing know-how—capabilities that outsourcing partners are well-positioned to provide.
Geographically, North America and Europe currently dominate the market thanks to their mature pharmaceutical industries and strong regulatory frameworks. However, the Asia-Pacific region is growing rapidly, driven by cost advantages, expanding technical capabilities, and increasing participation in global drug development programs.
The Shift Toward Integrated, End-to-End Services
One of the most important trends reshaping this market is the move toward integrated, end-to-end service offerings. CDMOs are no longer positioning themselves as narrow service providers focused only on formulation tasks. Instead, many are expanding into holistic development partners, covering everything from early-stage preformulation to clinical and commercial manufacturing support.
This strategic shift is clearly visible in industry investments and expansions. For example, Skyepharma Productions SAS announced the creation of a center of excellence for product formulation and development, signaling the industry’s move toward more comprehensive, integrated service models. Similarly, companies like Formulationbio have expanded into analytical services, particle size analysis, stability testing, and solid dosage form development, offering clients a one-stop solution for complex development needs.
For sponsors, this integration reduces coordination risk, improves development efficiency, and shortens timelines—critical advantages in a competitive, high-cost R&D environment.
R&D Investment: Fuel for Outsourcing Growth
Global investments in pharmaceutical research and development continue to rise, and this is directly boosting demand for outsourced formulation services. Governments and private companies alike are increasing R&D budgets to maintain innovation pipelines and stay competitive in fast-moving therapeutic areas.
For instance, the United Kingdom committed to a significant increase in public R&D investment, aiming to raise spending by 15% and reach around EUR 22 billion by 2024–2025. Such large-scale funding commitments signal a long-term focus on pharmaceutical and life sciences innovation, which in turn drives demand for specialized development services—including formulation outsourcing.
At the corporate level, the financial pressure is even more evident. Major pharmaceutical companies are spending unprecedented amounts on R&D. Merck & Co., for example, reported FY 2023 revenues of $60.1 billion, with R&D spending reaching $30.5 billion—accounting for more than half of its total revenue. Johnson & Johnson also invested a record $15.1 billion in R&D in 2023. These figures highlight why companies are increasingly looking to optimize costs and improve efficiency by outsourcing complex development activities.
Key Growth Drivers
1. Growing Requirement for Specialized Knowledge
Modern drug formulations are more complex than ever. Many pharmaceutical companies lack the in-house expertise and advanced technologies needed to handle sophisticated delivery systems, stability challenges, and bioavailability optimization.
A relevant example comes from the Indian pharmaceutical sector, where companies are increasingly targeting global markets, especially the United States. To succeed, they must comply with stringent regulatory guidelines, including those related to formulation development. Outsourcing partners with deep regulatory and technical expertise provide a clear advantage here, helping companies meet international standards while improving product quality and performance.
This rising need for specialized scientific and regulatory knowledge is a major reason why the formulation development outsourcing market is expected to continue its strong growth trajectory.
2. Increasing Adherence to Regulations
Regulatory requirements for pharmaceutical manufacturing and development are becoming stricter worldwide. Compliance now involves detailed documentation, quality systems, recall procedures, and rigorous validation processes.
In India, for example, updated production standards under Schedule M require companies to notify authorities about product defects, deterioration, and recalls—requirements that were not formally structured before. For many small and mid-sized manufacturers, meeting these standards internally is both costly and complex. Outsourcing to partners with proven regulatory expertise offers a practical solution and is expected to further support market expansion.
3. Rising Costs of Research and Development
Drug development is expensive, risky, and time-consuming. As R&D budgets grow, companies are under increasing pressure to allocate resources more efficiently. Outsourcing formulation development allows firms to convert fixed costs into variable costs, access specialized infrastructure without heavy capital investment, and focus internal teams on core strategic activities.
With R&D spending reaching record levels across the industry, this cost-efficiency argument is becoming one of the strongest drivers of outsourcing adoption.
Challenges in the Formulation Development Outsourcing Market
Communication Gaps and Project Delays
Outsourcing inherently involves collaboration across organizations, often across borders. Differences in time zones, language, and corporate culture can create communication gaps that lead to misunderstandings, delays, and rework.
Formulation development is highly detail-sensitive. Even small misalignments in technical requirements or project goals can result in costly setbacks. To mitigate these risks, sponsors must invest in strong governance structures, regular communication routines, and shared digital platforms to ensure transparency and alignment throughout the development process.
Limited Customization and Flexibility
Another challenge is the limited flexibility offered by some CDMOs that rely on standardized service packages. While these models can be efficient, they may not fully address the unique technical or regulatory needs of complex or innovative drug candidates, such as biologics or specialty therapies.
Sponsors working on highly specialized products must carefully select partners who can offer tailored solutions, scientific depth, and adaptive development strategies. Without this flexibility, companies risk suboptimal formulations or delayed development timelines.
Regional Market Insights
United States
The United States remains one of the most important markets for formulation development outsourcing. The country’s strong pharmaceutical and biotechnology ecosystem, combined with a rigorous regulatory environment, drives high demand for specialized formulation services. Outsourcing is particularly popular among small and mid-sized companies that lack internal R&D infrastructure but are working on complex therapies such as biologics and personalized medicines.
Germany
Germany stands out in Europe due to its strong research base, advanced infrastructure, and commitment to high quality standards. The country hosts numerous reputable CROs and CDMOs offering specialized preformulation and formulation services, particularly in oncology, neurology, and infectious diseases. Its strong regulatory framework further enhances its attractiveness as an outsourcing destination.
India
India is experiencing significant growth in formulation development outsourcing, supported by a skilled workforce, cost-effective services, and a robust pharmaceutical industry. Indian CDMOs serve both domestic and international clients, with strong demand in areas such as oncology, neurology, and infectious diseases. The increasing focus on complex formulations, including biologics, is further strengthening India’s position in the global market.
United Arab Emirates
The UAE is emerging as a strategic hub for pharmaceutical manufacturing and development, supported by government initiatives such as “Make it in the Emirates” and the broader “Operation 300bn” strategy. These policies aim to boost domestic production and reduce reliance on imports, creating a favorable environment for formulation development outsourcing. The country’s modern infrastructure and regulatory alignment with global standards add to its appeal.
Recent Industry Developments
The market is also being shaped by strategic partnerships and acquisitions. In May 2024, AGC Biologics and BioConnection announced a collaboration to offer end-to-end biopharmaceutical development and manufacturing services, combining development expertise with specialized aseptic filling capabilities.
In April 2024, CoreRx Inc. acquired Societal CDMO Inc. for USD 130 million, significantly expanding its capabilities in formulation research, early-stage production, clinical trial services, and commercial-scale manufacturing. These moves reflect a broader industry trend toward consolidation and capability expansion to meet growing client demands.
Market Segmentation Snapshot
By Service:
Formulation Development
Preformulation
By Formulation:
Oral
Injectable
Others
By Therapeutic Area:
Oncology
Infectious Diseases
Neurology
Hematology
Respiratory
Cardiovascular
Dermatology
Others
By End Use:
Pharmaceutical and Biopharmaceutical Companies
Government and Academic Institutes
Others
By Region:
North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Key companies active in the market include SGS S.A., Intertek Group, Recipharm, Lonza, Charles River Laboratories, Eurofins Scientific, Labcorp, and Thermo Fisher Scientific, among others.
Final Thoughts: Outsourcing as a Strategic Imperative
The Formulation Development Outsourcing Market, projected to grow from US$ 33.6 billion in 2024 to US$ 64.74 billion by 2033, is no longer just a support segment of the pharmaceutical industry—it is becoming a strategic pillar of modern drug development.
Rising R&D costs, increasing regulatory complexity, and the growing sophistication of therapies are pushing companies toward specialized external partners. At the same time, CDMOs are evolving into integrated solution providers, offering end-to-end services that align perfectly with the industry’s need for speed, efficiency, and quality.
Looking ahead to 2025–2033, outsourcing in formulation development is set to play an even more central role in shaping how new medicines are designed, tested, and brought to market—making it one of the most important enablers of the next generation of pharmaceutical innovation.






Comments
There are no comments for this story
Be the first to respond and start the conversation.