United States In Situ Hybridization Market Size and Forecast 2026–2034
Precision Diagnostics Drive Strong Growth in Tissue-Based Molecular Testing Across the U.S.
United States In Situ Hybridization Market Overview
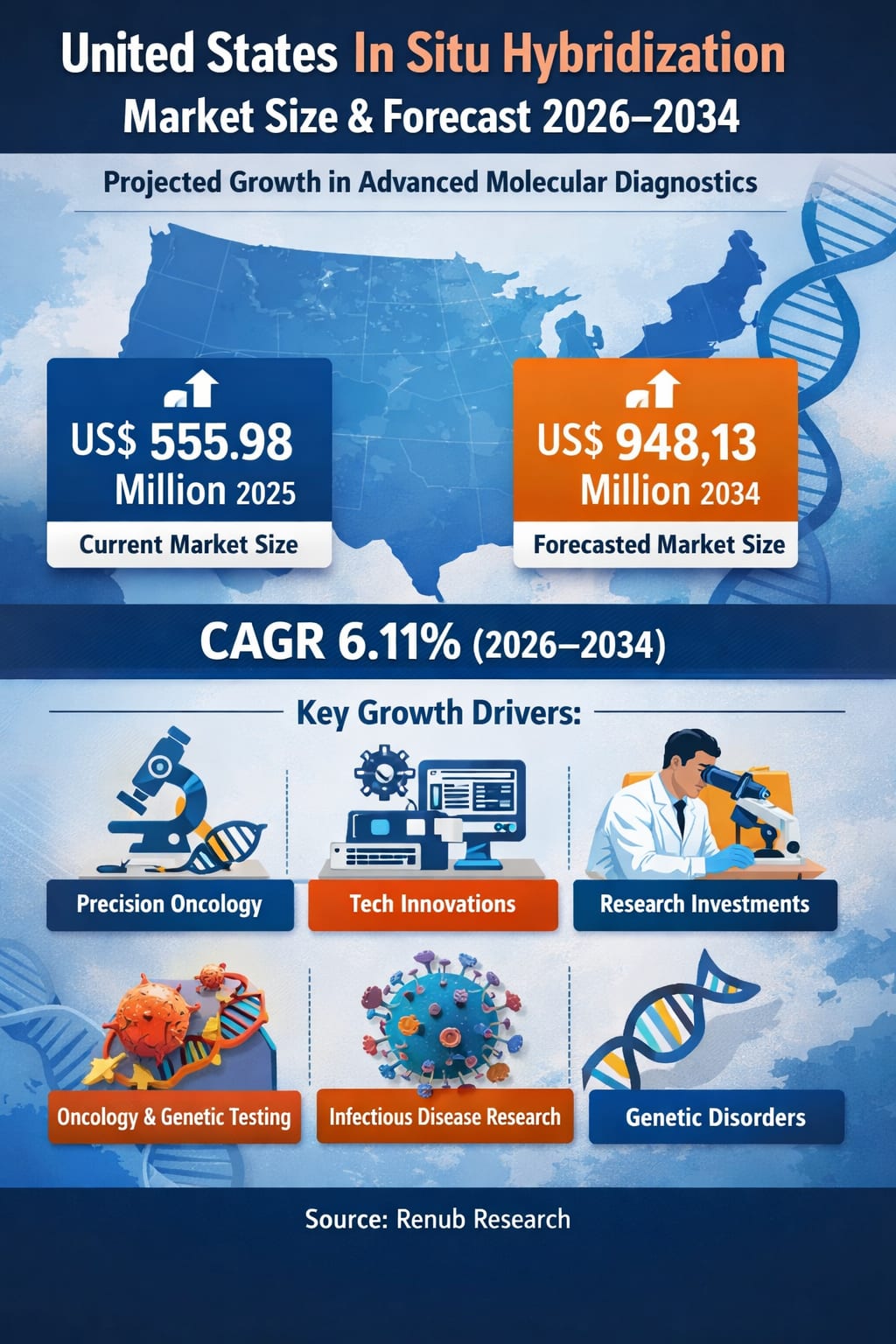
The United States In Situ Hybridization (ISH) market is witnessing steady and structurally strong expansion, driven by the rapid adoption of advanced molecular diagnostic techniques across clinical and research settings. According to Renub Research, the U.S. ISH market is projected to increase from US$ 555.98 million in 2025 to US$ 948.13 million by 2034, growing at a compound annual growth rate (CAGR) of 6.11% during 2026–2034.
This growth reflects the rising importance of tissue-based molecular diagnostics in oncology, genetic disease identification, infectious disease research, and translational medicine. As healthcare systems shift toward precision medicine and biomarker-guided therapies, ISH has become an indispensable tool for visualizing gene expression and chromosomal alterations directly within intact tissue architecture.
United States In Situ Hybridization Market Outlook
In Situ Hybridization is a highly sensitive molecular technique used to detect and localize specific DNA or RNA sequences within preserved cells or tissue sections. By using labeled probes that bind to complementary nucleic acid targets, ISH enables researchers and clinicians to observe molecular changes while maintaining spatial context. This capability distinguishes ISH from sequencing-only methods, which often lack anatomical resolution.
Variants such as Fluorescence In Situ Hybridization (FISH) and Chromogenic In Situ Hybridization (CISH) are extensively used in diagnostic pathology, particularly for cancer biomarker detection, chromosomal rearrangement analysis, and gene amplification studies. Advanced RNA-based methods such as RNAscope and hybridization chain reaction assays have further expanded ISH’s role in transcriptomic profiling at the single-cell level.
In the United States, the growing emphasis on early diagnosis, personalized therapies, and targeted drug development has positioned ISH as a cornerstone of molecular pathology workflows in hospitals, reference laboratories, and academic research centers.
Key Growth Drivers in the United States In Situ Hybridization Market
Rising Adoption of Precision Oncology and Biomarker-Guided Therapies
Precision oncology is a primary catalyst behind the expanding use of ISH in the United States. Modern cancer care increasingly relies on identifying actionable genetic alterations within tumor tissue to guide treatment selection. ISH techniques enable the detection of gene amplifications, deletions, translocations, and copy number variations that directly inform therapeutic decisions.
FISH and CISH assays are routinely employed for confirming biomarkers such as HER2 amplification, ALK and ROS1 rearrangements, and other clinically actionable targets. As oncology shifts away from generalized chemotherapy toward biomarker-driven regimens, the need for accurate, spatially resolved molecular assays continues to grow.
The approval of advanced ISH-based companion diagnostics further supports clinical adoption. In January 2025, F. Hoffmann-La Roche Ltd received U.S. FDA clearance for its VENTANA dual ISH mRNA probe cocktail, highlighting the expanding regulatory acceptance of ISH technologies in routine diagnostics.
Technological Advances in Automation, Imaging, and Probe Chemistry
Technological innovation has significantly enhanced the performance and accessibility of ISH assays. Automated slide staining systems have reduced manual variability while increasing throughput, making ISH more feasible for high-volume diagnostic laboratories. Improvements in probe design, fluorophore brightness, and signal amplification have expanded ISH applications to low-abundance RNA targets.
Digital pathology platforms now integrate whole-slide imaging, quantitative analysis, and artificial intelligence-assisted interpretation. These capabilities improve diagnostic consistency, enable remote consultation, and support large-scale clinical trials.
In October 2022, Bio-Techne Corporation expanded its RNAscope portfolio with automated co-detection assays, enabling simultaneous RNA and protein visualization within the same tissue section—an important advancement for translational research and biomarker discovery.
Expanding Investment in Molecular Pathology and Translational Research
Increased funding for molecular pathology, genomics research, and clinical trials is driving sustained demand for ISH in the United States. Federal research initiatives, academic grants, and pharmaceutical R&D investments continue to strengthen laboratory infrastructure and testing capacity.
Clinical trials increasingly require spatially resolved molecular endpoints, particularly for studying tumor heterogeneity and immune microenvironments. ISH plays a critical role in validating gene expression patterns within tissue context, complementing sequencing-based analyses.
In January 2025, Leica Biosystems announced a strategic investment in Indica Labs, underscoring growing industry focus on AI-enabled digital pathology and advanced biomarker interpretation.
Challenges in the United States In Situ Hybridization Market
High Cost of Equipment, Reagents, and Skilled Labor
Despite its clinical value, ISH remains a capital-intensive diagnostic modality. Automated stainers, high-resolution fluorescence microscopes, and digital scanners require substantial upfront investment. Reagents such as labeled probes, amplification kits, and quality control materials add to per-test costs.
Additionally, ISH demands highly trained histotechnologists and molecular pathologists for assay execution and interpretation. Workforce shortages and competitive labor markets further increase operational expenses, particularly for smaller hospitals and community laboratories.
Technical Complexity and Standardization Issues
ISH assays are sensitive to pre-analytical variables such as tissue fixation, processing, and section thickness. Variations in protocols across laboratories can lead to inconsistent results, complicating inter-laboratory comparability.
Interpretation of ISH results often requires expert judgment, especially in cases with heterogeneous or borderline signals. Multiplex assays add further complexity in spectral separation and data analysis. While automation and digital tools are improving consistency, full standardization remains a challenge.
United States In Situ Hybridization Market by Segment
Analytical Instruments Market
The U.S. ISH analytical instruments segment includes automated staining platforms, fluorescence microscopes, whole-slide scanners, hybridization systems, and image analysis software. Integrated workflows that combine staining, imaging, and analysis are increasingly preferred, as they reduce turnaround time and support regulatory compliance.
Demand is rising for compact, user-friendly instruments that enable mid-sized laboratories to internalize ISH testing without extensive infrastructure upgrades.
Fluorescence In Situ Hybridization Market
FISH remains the most widely used ISH technique in the United States due to its high sensitivity and clinical reliability. It is a standard diagnostic tool for hematologic malignancies, solid tumors, and genetic disorders. Advances in multiplex FISH and improved fluorophores continue to expand its diagnostic scope.
Chromogenic In Situ Hybridization Market
CISH offers colorimetric detection visible under standard brightfield microscopes, making it accessible to pathology laboratories without specialized fluorescence equipment. Its compatibility with routine histology workflows and long-term slide stability make it particularly valuable in community hospital settings.
Infectious Disease ISH Market
ISH plays a crucial role in infectious disease pathology by enabling direct visualization of pathogen nucleic acids within tissue samples. It is especially useful for organisms that are difficult to culture or when PCR results require histological correlation. ISH supports diagnosis in transplant medicine, neuropathology, and outbreak research.
Genetic and Rare Disorders ISH Market
For genetic and rare disorders involving mosaicism or tissue-specific gene expression, ISH provides essential spatial insight. It complements sequencing data by correlating molecular findings with anatomical context, supporting both diagnosis and research into disease mechanisms.
Regional Outlook: Key U.S. States
California
California leads the U.S. ISH market due to its concentration of academic institutions, biotechnology firms, and advanced cancer centers. Strong collaboration between life-science companies and digital pathology innovators supports early adoption of advanced ISH technologies.
New York
New York’s dense healthcare infrastructure and high patient volumes sustain robust demand for ISH in oncology, infectious disease diagnostics, and clinical trials. Large reference laboratories provide specialized ISH services across the Northeast.
Washington and Arizona
Washington benefits from a growing biotech ecosystem and research-driven demand, while Arizona’s expanding healthcare infrastructure supports steady growth through centralized testing models and telepathology collaborations.
Market Segmentation Summary
By Product:
Analytical Instruments; Probes, Kits & Reagents; Software & Services; Other Products
By Technique:
Fluorescence ISH (FISH); Chromogenic ISH (CISH); Amplified RNA-ISH; In-situ Sequencing
By Application:
Cancer Diagnostics & Research; Infectious Diseases; Genetic & Rare Disorders; Neurological Research
By End User:
Diagnostic Laboratories; Academic & Research Institutes; Pharma-Biotech & CROs
Competitive Landscape
Key companies operating in the U.S. In Situ Hybridization market include Thermo Fisher Scientific, Inc., Agilent Technologies, Inc., Merck KGaA, Bio-Rad Laboratories, Inc., NeoGenomics Laboratories, Inc., and Advanced Cell Diagnostics, Inc.. Companies are evaluated across product innovation, leadership, recent developments, SWOT analysis, and revenue performance.
Final Thoughts
The United States In Situ Hybridization market is positioned for sustained and resilient growth through 2034, supported by expanding applications in oncology, infectious disease diagnostics, and genetic research. As precision medicine continues to shape clinical decision-making, ISH’s ability to deliver spatially resolved molecular insights ensures its continued relevance and adoption.
With ongoing advances in automation, imaging, and probe chemistry—and increasing integration with digital pathology and AI—the role of ISH in the U.S. healthcare ecosystem will only deepen, reinforcing its importance as a foundational molecular diagnostic technology.
About the Creator
Sakshi Sharma
Content Writer with 7+ years of experience crafting SEO-driven blogs, web copy & research reports. Skilled in creating engaging, audience-focused content across diverse industries.






Comments
There are no comments for this story
Be the first to respond and start the conversation.